フランスのテレビ番組で、BMN-111の治験がニュースとなっています。本件は、ALPE FOUNDATIONの紹介情報です。(以前、セラピーの動画をご提供してくださったスペインの団体です。)
フェーズ2が始動時の26人の患者のうち、2名がフランスで治験に参加しておられます。当番組がアップせれたのは、去年の暮れ。既に当薬の6か月間の投与結果(2015年6月)は開示済みでした。以降、延長投与に進むものです。
全部フランス後なので、詳細はわかりませんが、ご家族やお子さんが夢と希望に満ちて胸膨らむ想いでいるのが、想像されますね。
It is a report program on TV in France about BMN-111. The movie image is listed the website of ALPE FOUNDATION.
The situation on this TV program is in Phase 2 that is 26 subjects enrolling US, UK australia and France. In that compay 2 of them are enrlling the clinical trial and descided to expand more 2 years (*)the period to try it. The program was listed the end of last December. (* according to other information, is might be 18 months)
This passaages below is quoted ALPE FOUNDATION.
============================================
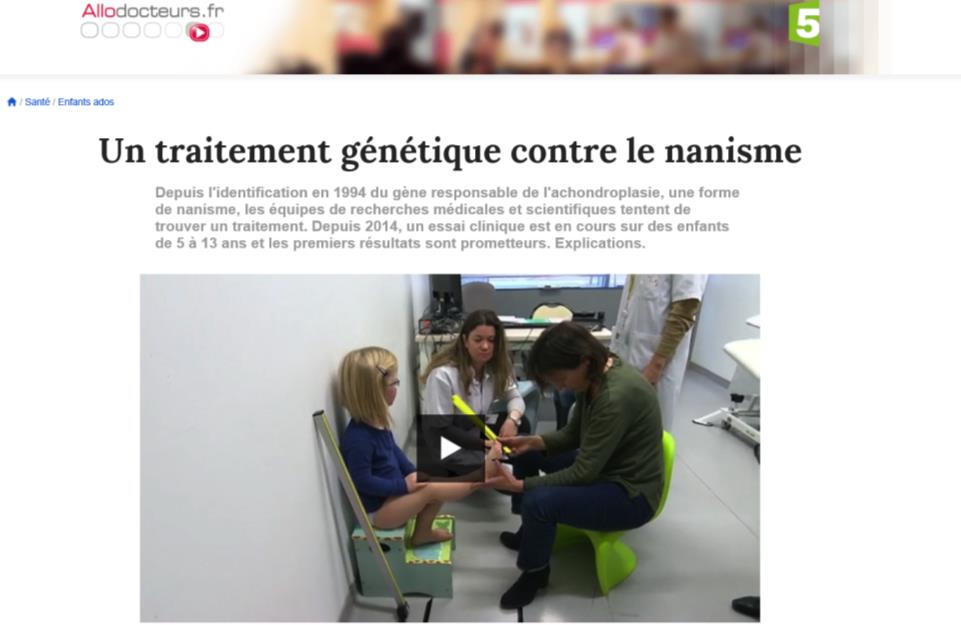
Since 1994 the identification of the gene responsible for achondroplasia, a form of dwarfism, medical and scientific research teams are trying to find a cure. Since 2014, a clinical trial is being conducted on children aged 5 to 13 years old and the first results are promising.
In achondroplasia, the FGFR3 gene is mutated and this receptor 3 of FGF´s (FGFR3) in chondrocytes (the cells in the growth plate) produces negative signals in large quantities. And this inhibits the natural growth rate. The treatment that is now been under study is actually another small protein with is another receptor, that is able to block inside the chondrocytes, the abnormally active FGFR3. It is a chain reaction that researchers first tested on human cells and mice.
Here you can see a video (in french)
The research was conducted in the laboratories of the Institute of genetic diseases, in Paris. Comparisons were made between the mice with achondroplasia treated with the protein and mice with achondroplasia untreated. The results were that the researchers found a particular difference in size between the femur of treated and untreated mice. The mice that were treated with the BMN-111 molecule had a longer femur than the untreated mice.
BMN-111 allowed the mice to grow by 10% in twenty days, with daily injections. For researchers, this experimental treatment is a step forward. “Not only it can modify cell proliferation, also it is able to modify cell differentiation. These are extremely important points because these cells do not proliferate well and do not differ much … and do not allow the bone to grow properly, “says Dr. Laurence Legeai-Mallet, director of research at the Institute of Genetic Diseases of Paris.
Twenty-six children in the world are under clinical study for this new drug. Two French children were selected for this clinical trial. Since the beginning of the experiment, the researchers observed an improvement in growth in all children. The laboratory that conducted the tests has also decided to extend testing for two years.
Based on an article of: FranceTvinfo
============================================