GTAはvosoritide(開発:Biomarin)をはじめとした臨床試験の動向をタイムリーに発信することを目指してきました。ここで2020年に実施中/実施見込みの治験薬リストについて、開発者や当局の直近の情報開示を踏まえ下表のとおり、個々の新薬の進捗、投与頻度、実施国、一部効能などについて 下表のとおり整理します。
GTA has been communicated timely to post trends of many efforts on clinical trials such as vosoritide. We summarized the following table the list of investigational drugs of API, administration, phase, location and efficacy currently are initiated / to be initiated in 2020, based on the latest information disclosure from developers and authorities.
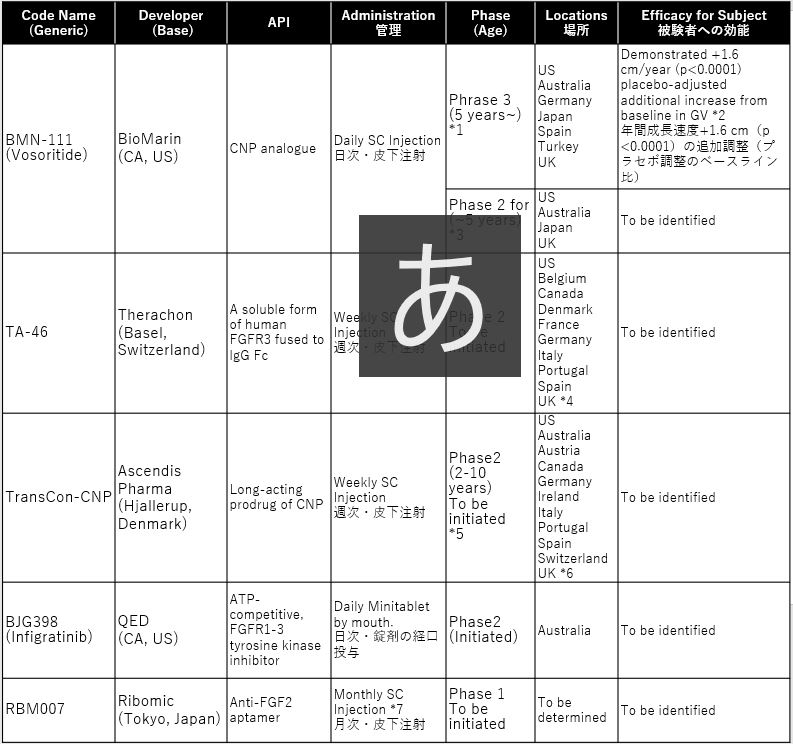
小児の軟骨無形成症を対象とした臨床試験 2020
List on Clinical trials for children with Achondroplasia in 2020
Sources:
*1: ClinicalTrials.gov as of 01/18/2020 “A Study to Evaluate the Efficacy and Safety of BMN 111 in Children With Achondroplasia”
https://clinicaltrials.gov/ct2/show/NCT03197766?term=111-301&draw=2&rank=1
*2: Fourth Quarter and Record Full-year 2019 Financial Results
*3: ClinicalTrials.gov as of 03/04/2020 “An Extension Study to Evaluate Safety and Efficacy of BMN 111 in Children With Achondroplasia”
https://clinicaltrials.gov/ct2/show/NCT03989947?term=111-206&draw=2&rank=2
*4: ClinicalTrials.gov as of 04/18/2019 “Observational Study Investigating Clinical & Anthropometric Characteristics of Children With Achondroplasia”
https://clinicaltrials.gov/ct2/show/NCT03794609?term=TA46&draw=2&rank=1
*5: ClinicalTrials.gov as of 09/11/2019 “A Dose Escalation Trial Evaluating Safety, Efficacy, and Pharmacokinetics of TransCon CNP Administered Once Weekly in Prepubertal Children With Achondroplasia”
https://clinicaltrials.gov/ct2/show/NCT04085523?cond=achondroplasia&draw=2&rank=3
*6: ClinicalTrials.gov as of 09/06/2019 “A Multi-center, Longitudinal, Observational Study of Children With Achondroplasia”
https://clinicaltrials.gov/ct2/show/NCT03875534?cond=achondroplasia&draw=2&rank=2#contacts
*7: Presentation Materials at the J.P. Morgan Healthcare Conference 2020
Key findings;
vosoritideは、既に第3相試験の全被験者が、2年目の投与段階に入っており、すべてのコーホートが実薬に切り替わっています。有害事象は報告されていません。効果について、健常児の成長曲線には届かないものの+1.6cm/yearの追加成長を記録しています。一方、考慮すべきは、日次の皮下注射が想定される患者負担、および最初の承認は5歳もしくは6以上の小児に限られることです。乳幼児の小児については、開始されたばかりの第2相試験(111-206)の終了後に承認申請に入ることとなります。
TA-46およびTransCon-CNPは、患者負荷の低い週次での投与を想定した第2相試験を2020年に開始する見込みです。
infigratinib について、ATP競合/FGFR1-3チロシンキナーゼ阻害剤は、未だ軟骨無形成症での投与実績はありませんが、癌領域ではいくつかの臨床試験での使用実績があります。
RBM007は、これら開発薬の後続となるものの、最も患者負担の低い月次での投与が想定されています。
Vosoritide has already been proceeded the second phase of administration for all subjects in the Phase 3 trial, and all placebo cohorts have switched to active drug. No Adverse events were reported. The effect is identified +1.6/year additional height although it does not reach the growth curve of healthy. On the other hand, it is important to consider that daily SC injections are assumed high patients burden. And initial approval is limited to children 5 years or older. For infants and young children, it will be applied for approval after the phase II trial (111-206) which has just started.
TA-46 and TransCon-CNP will only begin a phase II study in 2020, envisioning weekly dosing with low patient burden.
For infigratinib, an ATP-competitive / FGFR1-3 tyrosine kinase inhibitor has not yet been administered in achondroplasia, but it has been used in several clinical trials in the area of oncology.
RBM007 is expected to be a monthly follow-up to these developmental drugs which is the least patient burden even in most behind.


