2021年11月18日(木)(日本時間)、RIBOMICは、機関投資家・アナリスト向け2022年3月期第2四半期決算説明会において、軟骨無形成症の小児に対し開発中の治療薬「RBM-007」の第1相試験の結果および今後の開発スケジュールを発表しました。軟骨無形成症の小児に対し開発中の治療薬「RBM-007」に関して新しく開示された情報を抜粋します。
※本資料は、関する軟骨無形成症の正式言語は日本語であり、その内容、解釈および補足情報については正式言語が優先します。詳細はSource をご参照ください。
On Thursday, November 18, 2021, RIBOMIC announced the results of a Phase 1 trial of the drug “RBM-007” under development for children with achondroplasia and future development schedules at their second-quarter financial results briefing for institutional investors and analysts.
* The official language of this document is Japanese, so this language has
priority over its content and interpretation. See Source for
details.
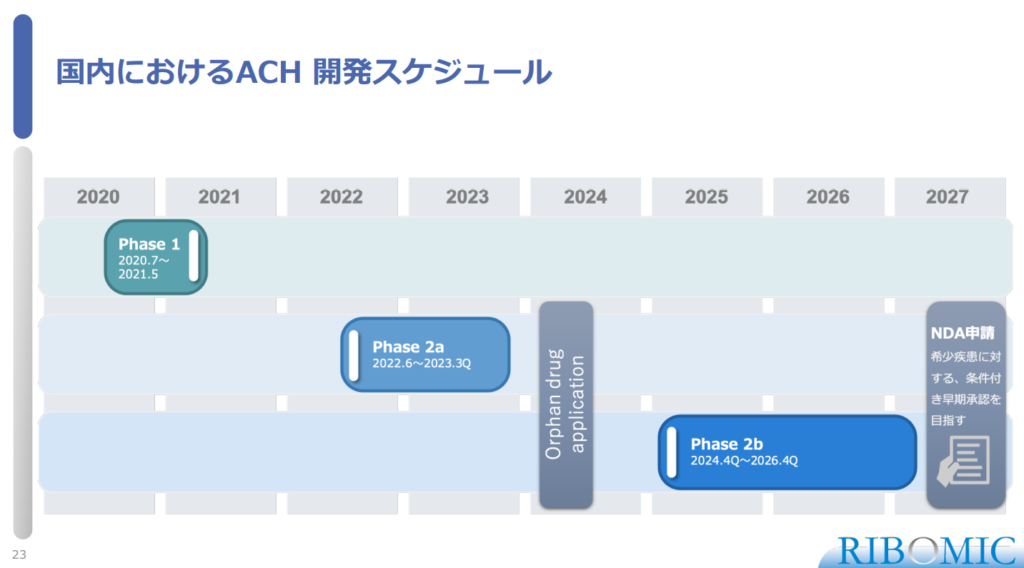
〈Development Schedule for Achondroplasia (RBM007) in Japan〉
- Phase 1 study was conducted for July, 2020- May, 2021.
- Phase 2a study will be conducted June 2022-3Q, 2023 and 2b study will be 4Q, 2014 – 4Q 2026.
- NDA application that is expected as conditional early approval for rare disease will be conducted in 2027
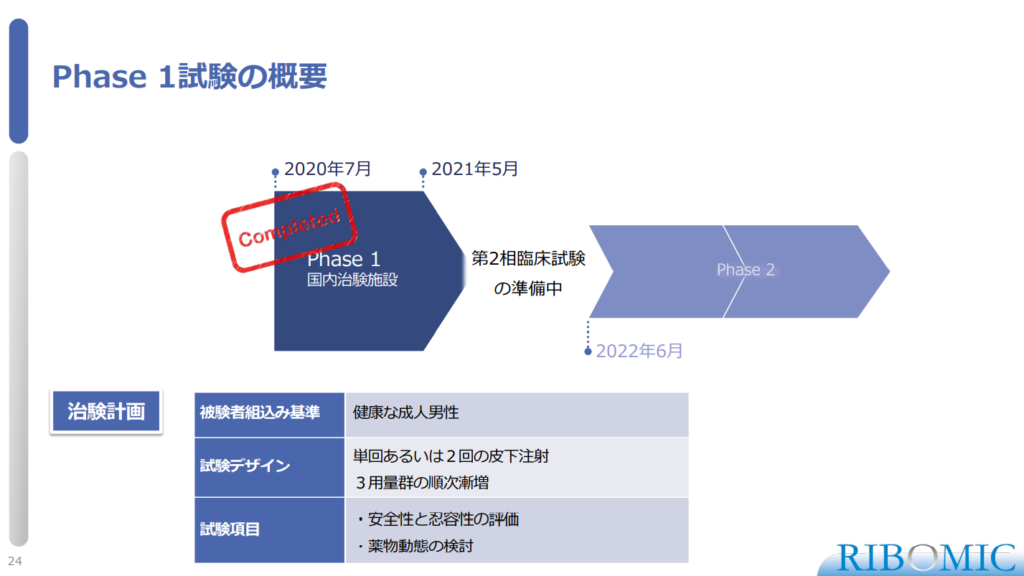
〈Overview of Phase 1 study〉
- Phase 1 design:
- Inclusion criteria: Healthy Adult Male
- Design: Single or two subcutaneous injections
Dose escalation in 3 dose groups
- Items: Evaluation of safety and tolerability
Examination of pharmacokinetics
- Currently it is under preparation for Phase 2 study to start from June, 2022.
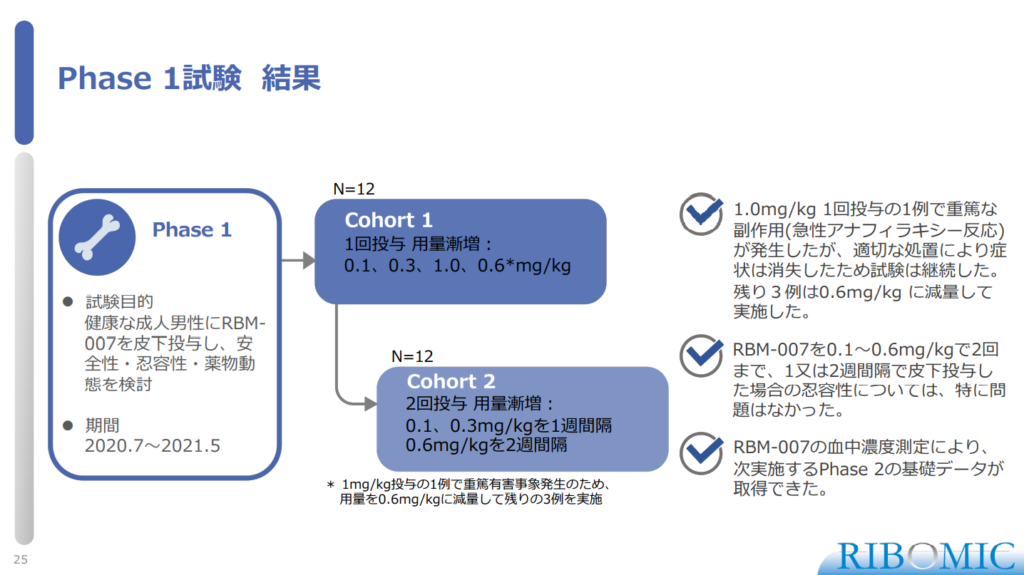
〈Result of Phase 1 study〉
- Serious side effects in a single dose of 1.0 mg/kg (acute anaphylactic reaction) However, the study was continued because the symptoms disappeared with appropriate treatment. The remaining 3 cases were reduced to 0.6 mg/kg.
- RBM-007 should be administered at 0.1-0.6 mg/kg up to 2 times, 1 patient at 2-week intervals. There were no adverse events with the tolerability of the case.
- By measuring the concentration in the water of RBM-007, they obtained the basic data for Phase 2 to be conducted the next.
(Cohort)
- Cohort 1
- N=12
- Single dose escalation: 0.1、03、1.0、0.6mg*/kg
*Due to the occurrence of serious adverse events in 1 case of 1 mg/kg administration, the dose was reduced to 0.6 mg/kg and the remaining 3 groups were conducted.
- Cohort 2:
- N=12
- 2 doses escalation: weekly 0.1, 03mg/kg and bi-weekly 0.6mg/kg
Source: RIBOMIC website


